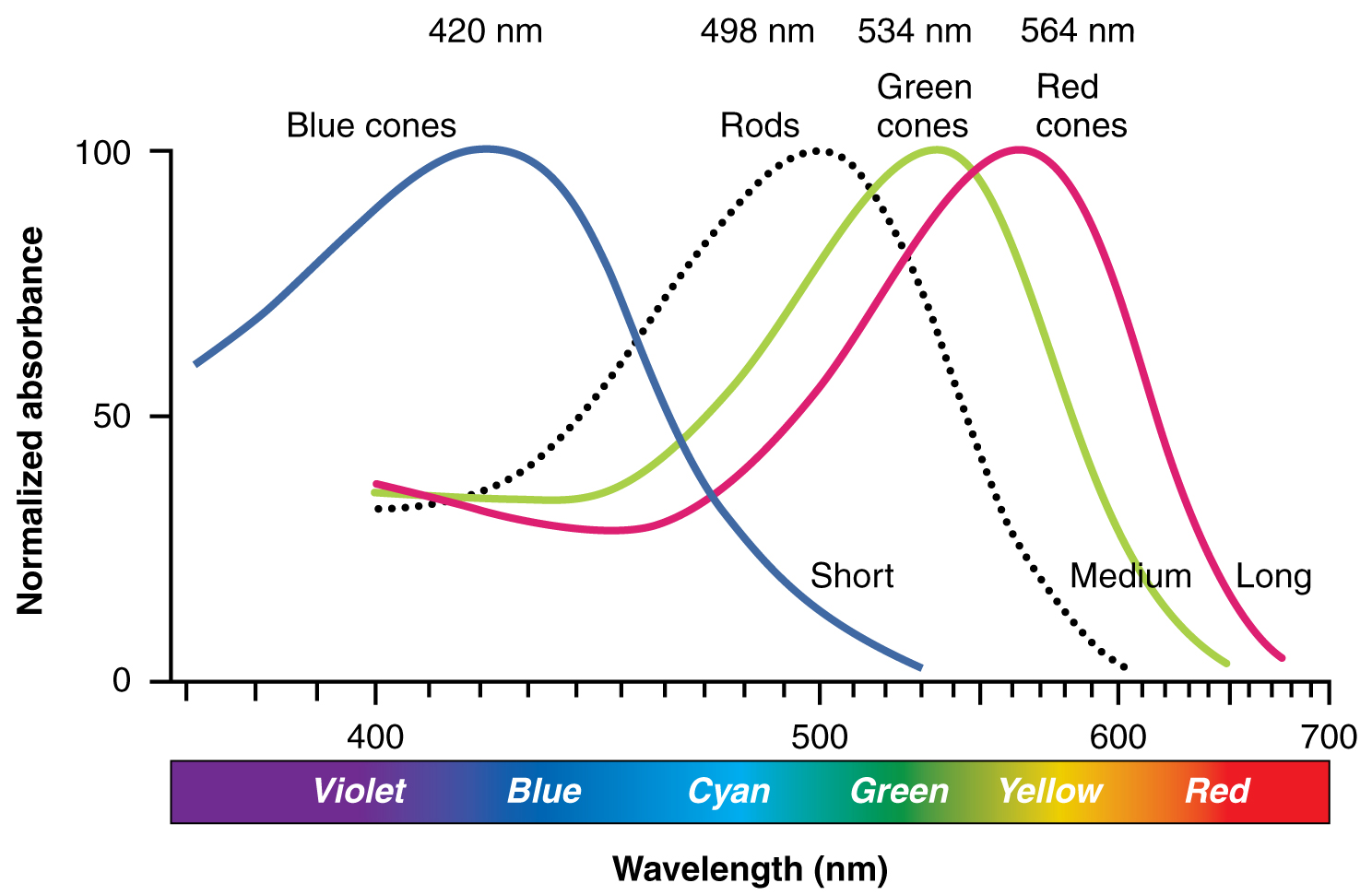
Sensory Perception · Anatomy and Physiology
Absorbance refers to the amount of light absorbed by a substance, while absorption is the process by which a substance takes in another substance or energy. Absorbance is measured using a spectrophotometer, which measures the amount of light absorbed by a substance.
PPT Absorbance spectroscopy PowerPoint Presentation, free download ID3102589
Main Difference - Absorbance vs. Transmittance. Absorbance and transmittance are two related, but different quantities used in spectrometry. The main difference between absorbance and transmittance is that absorbance measures how much of an incident light is absorbed when it travels in a material while transmittance measures how much of the light is transmitted.

Absorption spectroscopy of haemoglobin species Deranged Physiology
The main difference between them is that adsorption is the adhesion of particles onto a substance, while absorption involves mass transfer into another material. But, adsorption and absorption involve other differences as well. Here is a comparison of adsorption and absorption, a closer look at their definitions, and examples of each process.

Absorption Vs Adsorption The Engineering Concepts
Answer While absorption is the process of energy transfer of light into a medium that it passes through, absorbance is a quantification of exactly how much light is absorbed by the medium. Additional resources Absorption Spectrum Viewer What is the difference between absorbance and absorption?

Absorption spectra (Absorbance vs wavelength) of 5 NaOCl and dilutions... Download Scientific
This page titled 13.1: Transmittance and Absorbance is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by David Harvey. As light passes through a sample, its power decreases as some of it is absorbed. This attenuation of radiation is described quantitatively by two separate, but related terms: transmittance and..

Normalized absorbance and emission intensity spectra versus wavelength... Download Scientific
The term absorption refers to the physical process of absorbing light, while absorbance does not always measure only absorption; it may measure attenuation (of transmitted radiant power) caused by absorption, as well as reflection, scattering, and other physical processes.
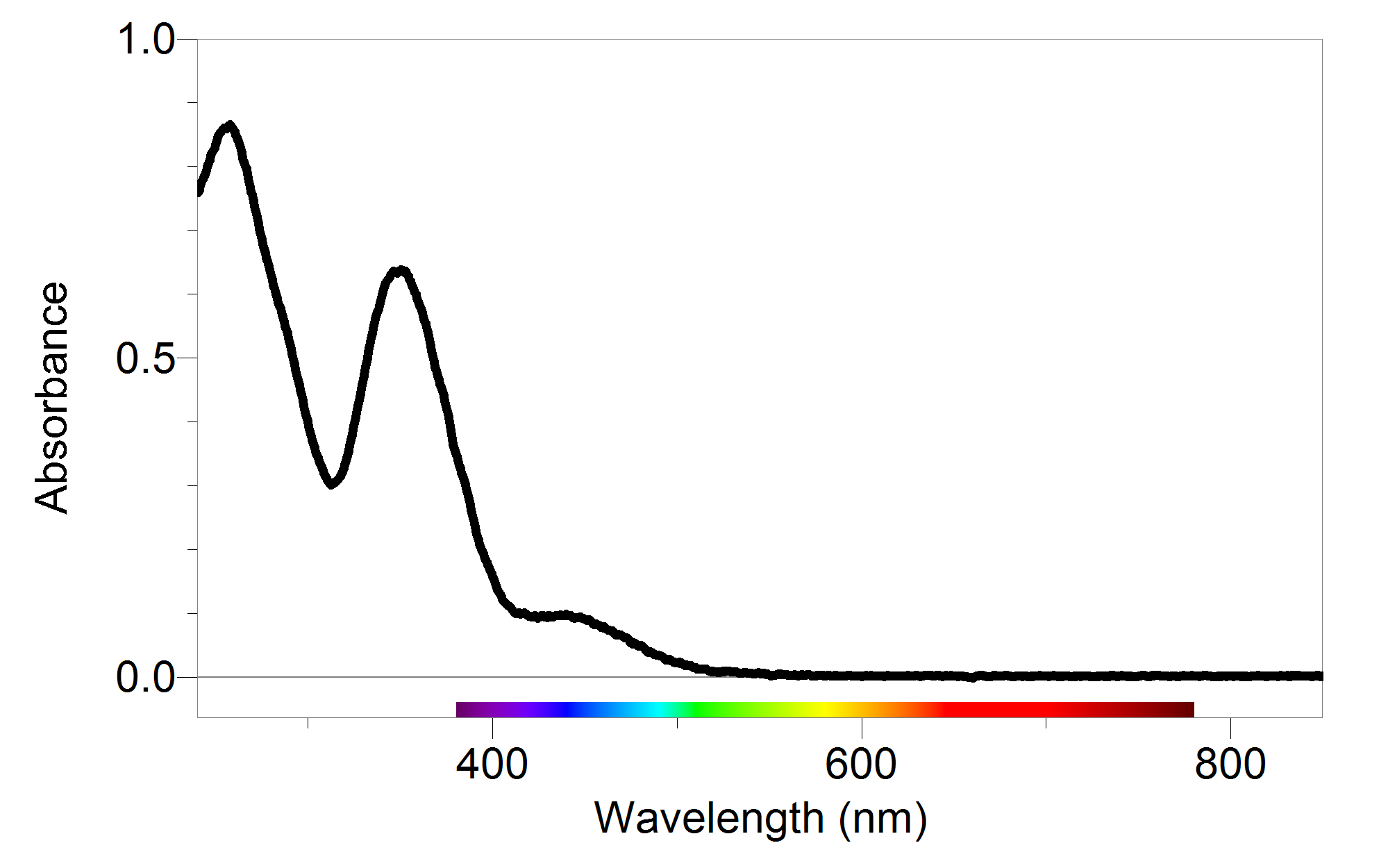
Decoding Your Absorbance Readings Vernier
What's the difference between Absorption and Adsorption? Absorption is the process in which a fluid is dissolved by a liquid or a solid (absorbent). Adsorption is the process in which atoms, ions or molecules from a substance (it could be gas, liquid or dissolved solid) adhere to a surface of the adsorbent. Ads.
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments
The result is an absorbance spectrum that shows the intensity of emission as a function of wavelength. As is the case for emission spectra, absorbance spectra range from narrow lines to broad bands. The atomic absorption spectrum for Na is shown in Figure 6.4.4 6.4. 4, and is typical of that found for most atoms.

UV absorption curve comparing the absorbance vs wavelength (λ) for... Download Scientific Diagram
The absorbance is directly proportional to the concentration (\(c\)) of the solution of the sample used in the experiment. The absorbance is directly proportional to the length of the light path (\(l\)), which is equal to the width of the cuvette. Assumption one relates the absorbance to concentration and can be expressed as \[A \propto c.
Solved Absorbance Vs. wavelength Absorbance 0 100 200 500
You will be applying Beer's law to calculate the concentration. The equation for Beer's law is: A = εmCl. (A=absorbance, εm = molar extinction coefficient, C = concentration, l=path length of 1 cm) You should have a data set which was used to create a standard curve. The graph should plot concentration (independent variable) on the x-axis and.

UVvis absorption spectra of absorbance versus wavelength from 200e750... Download Scientific
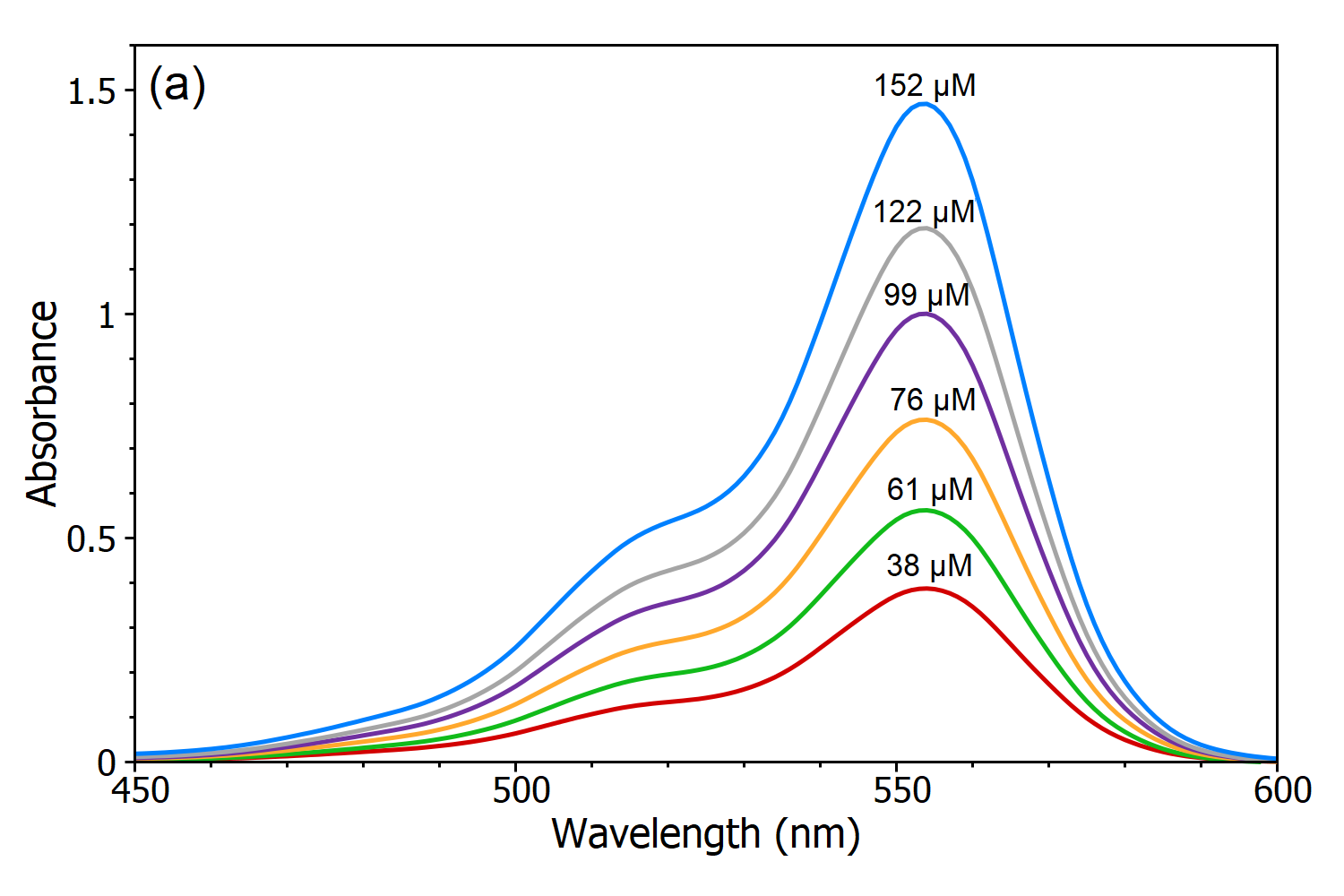
Solutions Absorbance vs Concentration Absorbance vs Concentration Introduction According to Beer's Law, A = εLc, a substance's concentration and absorbance are directly proportional under ideal conditions: a high-concentration solution absorbs more light. In comparison, a low-concentration solution absorbs less light.
shows the transmission and absorption spectra of the prepared silicon... Download Scientific
4.4: UV-Visible Spectroscopy. Ultraviolet-visible (UV-vis) spectroscopy is used to obtain the absorbance spectra of a compound in solution or as a solid. What is actually being observed spectroscopically is the absorbance of light energy or electromagnetic radiation, which excites electrons from the ground state to the first singlet excited.

(a) Absorbance vs. Wavelength spectrum and (b) Dependence of absorption... Download Scientific
Absorbance vs. Transmittance. The absorbance value can range from 0 to infinity, where a higher value indicates a greater absorption of light. Absorbance is commonly used to quantify the concentration of a substance in a solution, as it follows Beer-Lambert's Law, which states that absorbance is directly proportional to the concentration and.

Adsorption vs Absorption Differences and Examples
Absorption spectroscopy is spectroscopy that involves techniques that measure the absorption of electromagnetic radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this.

Absorbance vs wavelength for S1, S2, S3 and S4. Inset is for pure ZnS... Download Scientific
Spectrophotometers are a unique set of instruments made to quantitatively measure absorption of visible and ultraviolet (UV-Vis) light or emission of fluorescence compounds. Absorbance approaches directly measure the light absorbed by a sample at a specific wavelength, where the absorbed light measurement is proportional to the concentration of the sample.
️ Absorbance wavelength graph. Absorbance. 20190218
Transmittance is related to absorption by the expression: \(Absorbance (A) = - log(T) = - log(\dfrac{I_t}{I_o})\) Where absorbance stands for the amount of photons that is absorbed. With the amount of absorbance known from the above equation, you can determine the unknown concentration of the sample by using Beer-Lambert Law. Figure 5.